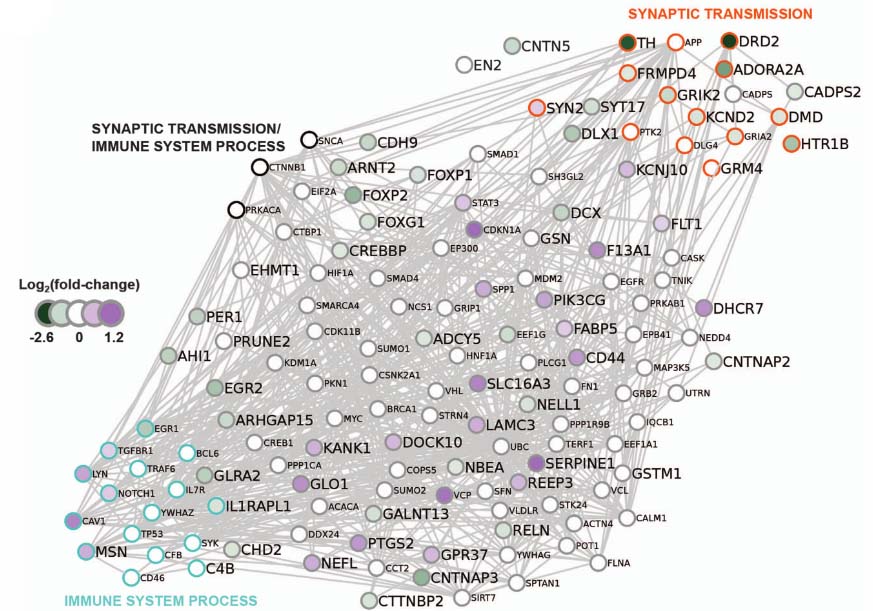
 Figure 3C
Figure 3C
 Figure 3C
Figure 3C
Autism spectrum disorder (ASD) is a neurodevelopmental condition with a clear, but heterogeneous, genetic component. Germline mutations in the tumor suppressor Pten are a well-established risk factor for ASD with macrocephaly, and conditional Pten mouse models have impaired social behavior and brain development. Some mutations observed in patients disrupt the normally balanced nuclear-cytoplasmic localization of the Pten protein, and we developed the Ptenm3m4 model to study the effects of a cytoplasm-predominant Pten. In this model, germline mislocalization of Pten causes inappropriate social behavior with intact learning and memory, a profile reminiscent of high-functioning ASD. These animals also exhibit histological evidence of neuroinflammation and expansion of glial populations by 6 weeks of age. We hypothesized that the neural transcriptome of this model would be altered in a manner that could inform human idiopathic ASD, a constitutional condition. Using total RNA sequencing, we found progressive disruption of neural gene expression in Ptenm3m4 mice from 2–6 weeks of age, involving both immune and synaptic pathways. These alterations include downregulation of many highly coexpressed human ASD-susceptibility genes. Comparison with a human cortical development coexpression network revealed that genes disrupted in Ptenm3m4 mice were enriched in the same areas as those of human ASD. Although Pten-related ASD is relatively uncommon, our observations suggest that the Ptenm3m4 model recapitulates multiple molecular features of human ASD, and that Pten operates far upstream of common pathways within ASD pathogenesis.